Dermatology
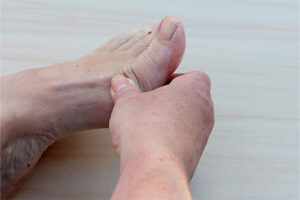
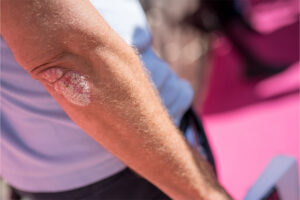
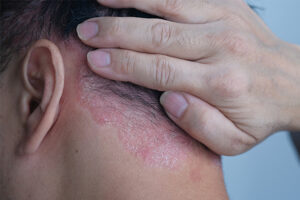
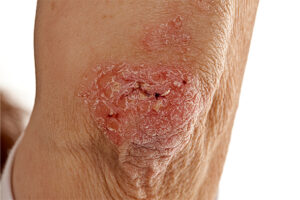
Plaque Psoriasis
Identifying Biomarkers of Treatment Response in Plaque Psoriasis
Prognostic biomarkers of disease progression in plaque psoriasis have been the focus of recent research, with a goal of potentially identifying patients who may respond to specific therapies and patients who may have varying clinical courses. The ability to predict treatment response may help clinicians choose the best initial therapy for an individual patient with plaque psoriasis.
The idea of identifying biomarkers to guide decision making in plaque psoriasis treatment is a promising one. It is likely that we will eventually identify a group of biomarkers as opposed to a single biomarker. Because no reliable biomarker has yet been identified, however, right now our greatest biomarkers and guides for clinical decision making are the patients themselves, both in terms of their clinical appearance and their satisfaction. But this is not always a linear relationship, as there will also be patients who would report not being satisfied with treatment regardless of how much clearance had been achieved. Similarly, biomarkers, like any test, would therefore be just 1 metric used for making treatment decisions.
There is currently a lot of pressure to identify a biomarker that would indicate the best treatment option for an individual patient. This is partially because we have so many effective treatment options available. It is a good challenge to have, but, when starting patients on biologic therapy, choosing 1 agent over another can be very difficult, especially for residents and dermatologists who are newer to practice.
From the perspective of optimal initial treatment selection, it could be helpful to have a biomarker that gives us insight into how a patient is doing systemically and how their plaque psoriasis is affecting them. That said, I also think that, ultimately, the patient is going to guide us when we need to dose escalate, change treatments, or add on to their existing regimen. My patients who are taking small-molecule inhibitors, biologics, or a combination of therapies are on them because that is what they need for successful treatment. Would having a blood test change the way that I approached those treatment choices? I am not sure that it would. With that in mind, we would also need to be aware of any future biomarkers that are incurring excessive costs that are passed along to the patient and the health care system.
Using biomarkers for the purpose of selecting a treatment option for an individual patient with plaque psoriasis is not yet mature. There are several companies that are working on developing such tests. These tests would essentially sample the transcription of genes or the presence of specific proteins in patients with psoriasis and, through an algorithmic process, assign a probability of treatment success with either TNF blockers, IL-17 inhibitors, IL-12/23 inhibitors, or IL-23 inhibitors. That type of testing potentially could be quite accurate and helpful because it could mitigate the need to switch therapies later by providing information to help choose the best therapy for the individual patient up front.
However, we still do not have a blood test that can tell us, for example, whether a patient has an elevation or a depression of a specific protein that could inform treatment selection. That said, such a test might not be necessary. The currently available drugs are very effective, and the newest agents will work for most patients. If we already have an 80% to 90% success rate with existing therapies, do we need an additional test to help inform treatment? It is likely that many providers in the clinical setting would think of this issue in the same way.
I agree with the global thoughts shared by Dr Friedman and Dr Strober on this topic. And this idea of biomarkers for plaque psoriasis started with the use of drugs such as alefacept, which worked very well for a very small subset of patients. We wanted to identify those small subsets of patients who would benefit from this therapy, if we could. Since then, the biologic therapies that are available for patients with psoriasis have evolved dramatically, and now the majority of patients will see a positive clinical benefit when using newer biologic therapies. I personally find that approximately 90% of patients will have an excellent response. It is hard to imagine that the widespread use of a biomarker is going to provide additional useful information about who is going to respond to these treatments.
With such a high treatment success rate overall, it may be worthwhile to consider the following question: When patients do not improve on treatment, is it due to the biology of their psoriasis or to poor adherence? I believe that medication adherence is often an important contributor to a lack of clinical response, and understanding your patient and targeting barriers to adherence may help lead to a positive clinical outcome.
Corbett M, Ramessur R, Marshall D, et al. Biomarkers of systemic treatment response in people with psoriasis: a scoping review. Br J Dermatol. 2022;187(4):494-506. doi:10.1111/bjd.21677
Cruz CJG, Yang CC. Clinical application of serum biomarkers for detecting and monitoring of chronic plaque psoriasis. Front Mol Biosci. 2023;10:1196323. doi:10.3389/fmolb.2023.1196323
Hagino T, Saeki H, Kanda N. Biomarkers and predictive factors for treatment response to tumor necrosis factor-alpha inhibitors in patients with psoriasis. J Clin Med. 2023;12(3):974. doi:10.3390/jcm12030974
Pourani MR, Abdollahimajd F, Zargari O, Shahidi-Dadras M. Soluble biomarkers for diagnosis, monitoring, and therapeutic response assessment in psoriasis. J Dermatolog Treat. 2022;33(4):1967-1974. doi:10.1080/09546634.2021.1966357
Ramessur R, Corbett M, Marshall D, et al. Biomarkers of disease progression in people with psoriasis: a scoping review. Br J Dermatol. 2022;187(4):481-493. doi:10.1111/bjd.21627