Oncology
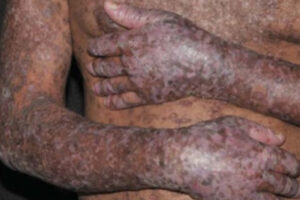
Chronic Graft-versus-Host Disease
Chronic Graft-versus-Host Disease: Clinical Application of Regulatory T Cells
Ongoing immune dysregulation following transplant may contribute to the development of chronic graft-versus-host disease (cGVHD). Regulatory T cells (Tregs) help maintain immune system balance by downregulating immune responses and have been found to be deficient in patients with cGVHD. Although there are currently no drugs to specifically expand Tregs, this is an area of ongoing interest and research.
Following a transplant, and for reasons that are not totally clear to us yet, a lot of immune dysregulation occurs within transplant recipients. Healthy individuals have a state of immunological balance, poised to react when challenged but not to excessively react. In GVHD, however, one key principle is that there is ongoing immune activation without proper regulation. One of the key ways that our immune system works is through certain cell populations that downregulate immune responses to maintain a healthy balance in the immune system. We have learned that there are defects and deficiencies in key cell populations that help regulate the immune system in patients who develop GVHD, including a cell population called Tregs.
Tregs are very important and have been found to be deficient in patients with cGVHD. This suggests that if you could increase the number of Tregs, you may be able to impact the disease burden. Unfortunately, we have not yet found an easy way to do that. No pharmacologic therapies have been identified that will specifically expand Tregs. That is an area of great interest because of its relevance in multiple disease states, including autoimmune disorders and others that are also impacted by a Treg deficiency. Probably the closest thing that we have is low-dose IL-2. Because Tregs express a very high-affinity IL-2 receptor, they respond to low doses of IL-2. However, IL-2 is certainly not specific for Tregs, and it can impact a variety of immune cells, which makes dosing difficult. Although some evidence suggests clinical response from the administration of exogenous IL-2, it has not been optimal. This is also a drug with a lot of side effects, and it has to be specially formulated and injected, so it is not easy to administer.
Could Tregs be selectively transferred from the donor? While theoretically possible, it is technically still very difficult. This is an area of research that I think has merit to try to study and develop further, but it is really in its infancy. We just do not have the tools yet to separate Tregs out in an effective way and to see if they will result in better patient responses. Another challenge is that we currently do not have very good animal models for cGVHD that would allow us to do the preclinical and pathophysiological work to give validity to this concept.
I think that this is where we are right now in the study of Tregs in patients with cGVHD. Clinical trials assessing newer therapies for cGVHD have begun to collect data regarding their effects on the Treg compartment, since there is increased awareness that Tregs may be an important mediator of disease. Some of us hope that Treg dysregulation is fundamental to the defect occurring in diseases such as acute GVHD, cGVHD, and autoimmune disorders. If we could restore immune balance, that may give a better clinical response and may allow patients to feel much better.
Chen Y, Xu Y, Wu D. Advances in graft-versus-host disease prevention strategies: latest updates from the 2022 ASH annual meeting. Exp Hematol Oncol. 2023;12(1):59. doi:10.1186/s40164-023-00425-y
Guo WW, Su XH, Wang MY, Han MZ, Feng XM, Jiang EL. Regulatory T cells in GVHD therapy. Front Immunol. 2021;12:697854. doi:10.3389/fimmu.2021.697854
Johnston L, Armstrong R, Baker J, et al. A phase I study of donor regulatory T cells as treatment for steroid dependent/refractory chronic graft versus host disease [abstract 722]. Abstract presented at: 64th American Society of Hematology Annual Meeting and Exposition; December 9-12, 2023; San Diego, CA.
Whangbo JS, Antin JH, Koreth J. The role of regulatory T cells in graft-versus-host disease management. Expert Rev Hematol. 2020;13(2):141-154. doi:10.1080/17474086.2020.1709436
Whangbo JS, Nikiforow S, Kim HT, et al. A phase 1 study of donor regulatory T-cell infusion plus low-dose interleukin-2 for steroid-refractory chronic graft-vs-host disease. Blood Adv. 2022;6(21):5786-5796. doi:10.1182/bloodadvances.2021006625