Oncology
Chronic Graft-versus-Host Disease
ROCK2 and Its Role in Chronic Graft-versus-Host Disease
In chronic graft-versus-host-disease (cGVHD), the ROCK2 signaling pathway regulates the balance between Th17 cells and regulatory T cells and controls profibrotic signaling. The ROCK2 inhibitor belumosudil is a novel agent that can provide meaningful benefits to patients with refractory cGVHD.
In cGVHD, the ROCK2 signaling pathway is integral in controlling the balance between Th17 cells and regulatory T cells and in regulating profibrotic signaling. ROCK2 inhibition provides clinical benefit to patients, which is not always the case when agents are developed for cGVHD.
Therefore, it is highly gratifying that belumosudil, a ROCK2 inhibitor, can provide meaningful benefits to a significant proportion of patients with refractory cGVHD. As patients move forward in their treatment course, we hope that their responses will be maintained or improved. That would really transform the prognosis of cGVHD, both for the field itself and for the patients who have these potentially debilitating conditions.
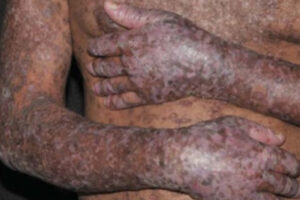
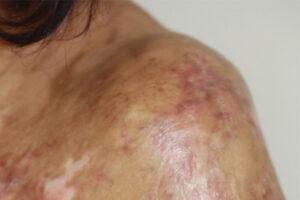
The availability of belumosudil has been a paradigm shift in the management of cGVHD because it means that we now have an agent that inhibits a known mechanism for the development of fibrosis. In clinical trials, belumosudil significantly improved and reversed sclerotic manifestations in patients whose cGVHD failed multiple lines of treatment. This is particularly meaningful for patients whose cGVHD affects their lungs, since a 30% response rate could mean the difference between being dependent on supplemental oxygen while walking vs being oxygen independent and able to better enjoy activities of daily living.
Clinical transplant specialists have recognized that some patients do really well and have no signs of cGVHD until we start tapering their immune suppression; within 1 month of them discontinuing immune suppression, some patients develop mouth sores, rashes, and other lesions. Ideally, we would have biomarkers to determine each patient’s level of immune suppression and predict flares. In cases where it seems like a patient will flare, we may want to put them on another agent, recognizing that long-term immunosuppressive therapy with calcineurin inhibitors and steroids is not ideal. Therefore, the ability to reduce or discontinue these drugs and replace them with an agent such as belumosudil will help raise the bar in the management of cGVHD, particularly in reducing relapse.
The clinical development of a ROCK2 inhibitor is a beautiful example of the successful use of preclinical models and evidence to identify a promising agent for cGVHD that demonstrates efficacy in clinical trials. Leveraging the substantial scientific underpinnings of our field led to the development of a clinically effective agent for a complicated and difficult problem. My only concern is the quality of the clinical evidence. Agents that are currently used for the management of cGVHD have different levels of evidence to support their use, from smaller phase 2 studies to larger phase 3 trials. I think that it is important to use high-quality evidence as we evaluate different treatment strategies moving forward.
There is a lot more to learn about these agents, especially as we have no data comparing them and no data about the use of agents in combination, but I think that the development of belumosudil is an excellent example of studying the science behind cGVHD and using a rational drug development process to successfully develop a drug.
Ali F, Ilyas A. Belumosudil with ROCK-2 inhibition: chemical and therapeutic development to FDA approval for the treatment of chronic graft-versus-host disease. Curr Res Transl Med. 2022;70(3):103343. doi:10.1016/j.retram.2022.103343
Cutler C, Lee SJ, Arai S, et al. Belumosudil for chronic graft-versus-host disease after 2 or more prior lines of therapy: the ROCKstar study [published correction appears in Blood. 2022;139(11):1772]. Blood. 2021;138(22):2278-2289. doi:10.1182/blood.2021012021
Jagasia M, Lazaryan A, Bachier CR, et al. ROCK2 inhibition with belumosudil (KD025) for the treatment of chronic graft-versus-host disease. J Clin Oncol. 2021;39(17):1888-1898. doi:10.1200/jco.20.02754
Taylor B, Cohen J, Tejeda J, Wang TP. Belumosudil for chronic graft-versus-host disease. Drugs Today (Barc). 2022;58(5):203-212. doi:10.1358/dot.2022.58.5.3400705
Zanin-Zhorov A, Blazar BR. ROCK2, a critical regulator of immune modulation and fibrosis has emerged as a therapeutic target in chronic graft-versus-host disease. Clin Immunol. 2021;230:108823. doi:10.1016/j.clim.2021.108823