Dermatology
Plaque Psoriasis
Choosing Systemic Agents for Psoriasis: What Clinicians Need to Know Now
A variety of systemic medications are available for the treatment of psoriasis, but there are many patient- and disease-related factors to take into account when choosing a systemic therapeutic option. A 4-part lecture presented at the 2023 Fall Clinical Dermatology Conference covered this important topic.
Following this presentation, featured expert Boni E. Elewski, MD, was interviewed by Conference Reporter Medical Writer Rick Davis. Dr Elewski’s clinical perspectives on this lecture are presented here.
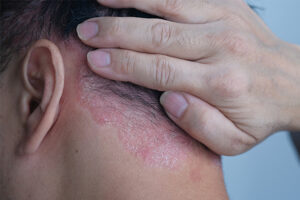
When deciding to start a patient on a systemic agent, there are several factors to consider, which were discussed in our 4-part presentation at the 2023 Fall Clinical Dermatology Conference. The first is the psoriasis severity, including the determination of moderate to severe disease. The way that this is determined can vary from patient to patient. It could be disease that covers greater than approximately 5% to 10% of a patient’s body surface area or disease that affects a particular part of the body. Further, if a patient has scalp, genital, face, or palm and sole disease that affects their quality of life—even if it covers less than 5% to 10% of their body—this would qualify as moderate to severe disease, and therefore this patient would be a candidate for a systemic agent.
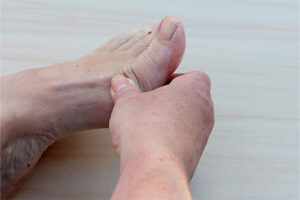
It is also important to consider whether a patient has psoriatic arthritis, which requires inquiring about symptoms of inflammatory joint disease, such as joint pain and stiffness upon awakening that improves as the day goes on. Another indication of psoriatic arthritis is the presence of severe nail psoriasis, including onycholysis with a red border, oil spots, and pitting. Generally, the worse a patient’s nails are, the more likely they are to have psoriatic arthritis and also severe skin disease.
The presence of inflammatory bowel disease (IBD) can also influence our decision-making process, as the interleukin-17 (IL-17) blockers are contraindicated in patients with this condition. Metabolic disease and high body weight are additional factors to take into account when making treatment choices because some patients who weigh more than approximately 200 pounds may require a drug that offers weight-based dosing or has data in patients with high body weight.
Once we have established the severity of the disease; the impact of the disease on the patient’s quality of life; and the presence or absence of joint disease, IBD, metabolic disease, and high body weight, we can decide on the best systemic option for that patient. For example, if they have joint disease and nail psoriasis, then IL-17 inhibitors are a good choice, but if they have IBD, then a TNF inhibitor or an interleukin-23 (IL-23) inhibitor would be preferred.
Some patients may prefer an oral medication rather than an injectable medication. Patients with mild psoriasis (eg, those with only small plaques on elbows and knees) could benefit from an agent such as apremilast, and this medication has been recently approved by the US Food and Drug Administration for all types of psoriasis. I often choose apremilast for patients who prefer an oral drug over a biologic injectable drug or have disease that is limited to the scalp or nails. The TYK2 inhibitor deucravacitinib is a new oral option that has shown superiority over apremilast in clinical trials. It is only minimally immunosuppressive, and it is very well tolerated. Deucravacitinib is technically classified in the JAK family, but it is not JAK-like and does not have a boxed warning like the other JAK inhibitors. It is an excellent option for patients with moderate to severe psoriasis who prefer an oral drug, including those who failed apremilast. Further, for patients with needle phobias or for those with challenging schedules, such as college students, we can choose either an oral drug or an injectable drug that requires fewer doses per year, such as the IL-23 blockers risankizumab (4 doses/year) or guselkumab (6 doses/year).
The biologic injectables can generally be given safely to patients of childbearing potential. Some studies have looked at discontinuing medications during the third trimester, so it is best to consult with the obstetrician to decide what is best for each patient. I did have one patient who discontinued their biologic during pregnancy, but her genital psoriasis flared to such an extent that the biologic needed to be restarted late in the second trimester. It is important to note that certolizumab pegol is the only agent that does not cross the placenta, and it is a safe choice in pregnant women; however, it is a TNF inhibitor and therefore has the side-effect profile that is associated with that class.
As outlined here, there are many factors to consider when selecting treatment for our patients with plaque psoriasis. The advent of new medications with unique mechanisms of action affords significant opportunities for better disease control with minimal toxicity. Nevertheless, we still have more to learn regarding long-term adverse events, impacts on future comorbidities, pediatric treatment, pregnancy and lactation, and treatment combinations for many of the newer biologic agents.
Elewski BE, Armstrong AW, Boh EE, Callis-Duffin K. Psoriasis & psoriatic arthritis—so now what do I do? Session presented at: 2023 Fall Clinical Dermatology Conference; October 19-22, 2023; Las Vegas, NV.
Feldman SR. Treatment of psoriasis in adults. UpToDate. Updated June 30, 2023. Accessed November 1, 2023. https://www.uptodate.com/contents/treatment-of-psoriasis-in-adults/print
Kontzias C, Chandy R, Feldman SR. Choosing systemic agents for psoriasis. Ann Pharmacother. 2023 Jun 21;10600280231170031. doi:10.1177/10600280231170031
Martin G, Young M, Aldredge L. Recommendations for initiating systemic therapy in patients with psoriasis. J Clin Aesthet Dermatol. 2019;12(4):13-26.
Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029-1072. doi:10.1016/j.jaad.2018.11.057
This information is brought to you by Engage Health Media and is not sponsored, endorsed, or accredited by the 2023 Fall Clinical Dermatology Conference.