Dermatology
Plaque Psoriasis
Plaque Psoriasis in the Real-World Pediatric Population
Plaque psoriasis can present at any age, including in pediatric patients. There are fewer US Food and Drug Administration (FDA)–approved treatment options available for the pediatric population than for adult patients, and real-world management requires the consideration of a wide range of clinical factors. Data presented at the Maui Derm Hawaii 2024 meeting provide additional insight into management options.
Following this presention, featured expert Bruce E. Strober, MD, PhD, was interviewed by Conference Reporter Medical Writer Rick Davis. Dr Strober’s clinical perspectives on this topic are presented here.
Approximately one-third of all patients with psoriasis present with symptoms prior to the age of 18 years. In my practice, I commonly first see patients starting at around 6 years of age, but some patients can have earlier or younger presentations (eg, starting as early as infancy).
It is possible that psoriasis can first present as somewhat of an eczematous phenotype in younger children and then evolve into the papulosquamous morphology that is typical of psoriasis. The parents might recall the patient having some papulosquamous dermatitis, perhaps primarily affecting the scalp, when they were an infant or a young child. And I have treated teenaged patients with psoriasis who described earlier symptoms that resembled eczematous dermatitis. Not uncommonly, pediatricians may initially mislabel psoriasis as eczema.
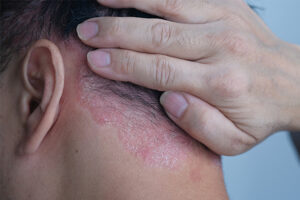
I find that psoriasis of the scalp is the most common presentation in pediatric patients, followed by psoriasis of the trunk and extremities. Certainly, the genitals can be affected, and that may more commonly present in children and adolescents. It is important to ask patients and their parents whether genital psoriasis is present. The children whom I treat also commonly have psoriasis that is refractory to topical therapy, which is usually started by their pediatrician. Additionally, in my opinion, patients with more severe pediatric psoriasis often also suffer from significant psychosocial disability. I have found that the parents are very eager to normalize the patient’s skin so that they can have more normal psychosocial development.
Children with psoriasis may also have psoriatic arthritis. The best analysis that I know of is from the early etanercept pediatric studies for patients with psoriasis, which documented that nearly 10% of enrolled patients also had psoriatic arthritis. That speaks to all aspects of psoriatic disease possibly also being present in pediatric patients. Therefore, questions surrounding psoriatic arthritis should be a part of the initial querying of the patient and their parents. There is probably a genetic lineage for psoriasis and psoriatic arthritis, and that is why you also want to ask about these conditions in the child’s parents and grandparents.
FDA-approved therapies for patients with pediatric psoriasis are more limited than what we have available for adult psoriasis, but that is changing with each passing year. We currently have 4 very effective biologics that are approved by the FDA for use in patients younger than 18 years: ustekinumab (down to the age of 6), etanercept (down to the age of 4), and the IL-17 inhibitors ixekizumab and secukinumab (both down to the age of 6).
You need to consider patient comorbidities when choosing therapy. The presence of psoriatic arthritis would make me lean toward using an IL-17 inhibitor or a TNF inhibitor. Further, some patients may have both inflammatory bowel disease and psoriasis, and this requires close coordination with their gastroenterologist to use therapies that may improve both conditions or to consider adding topicals to their biologic therapy, if needed. However, if I am treating straightforward psoriasis without psoriatic arthritis, my bias is toward using ustekinumab simply because I think that it works well. It also has a huge treatment advantage because of its less frequent dosing schedule (ie, every 12 weeks for pediatric patients after their 2 starter doses). You really want to minimize injections in children, for obvious reasons.
At Maui Derm Hawaii 2024, Fiorillo et al presented a poster on apremilast for the treatment of psoriasis in pediatric patients. The data in children were similar to what was seen in adults, which means that apremilast is a lower-efficacy therapeutic, with lower efficacy than a biologic. A benefit of apremilast is the lack of required laboratory monitoring. A negative, besides its low efficacy, is that it is a twice-daily oral medication, so adherence is likely to be an issue. And, as we know, for children, too, there are potential tolerability issues with apremilast, including nausea, headache, and diarrhea. For these reasons, I slate it behind biologic therapy for pediatric patients.
Afarideh M, Bartoletta K, Tollefson MM. Dermatologic manifestations in pediatric patients with inflammatory bowel disease. Pediatr Dermatol. 2024 Feb 7. doi:10.1111/pde.15538
Fiorillo L, Becker E, Belloni-Fortina A, et al. Apremilast for the treatment of psoriasis in special areas in pediatric patients in the SPROUT study. Poster presented at: Maui Derm Hawaii 2024; January 22-26, 2024; Wailea, HI.
Hebert AA, Browning J, Kwong PC, Duarte A, Price HN, Siegfried E. Diagnosis and management of pediatric psoriasis: an overview for pediatricians. J Drugs Dermatol. 2023;22(8):742-753. doi:10.36849/jdd.7531
Menter A, Cordoro KM, Davis DMR, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients [published correction appears in J Am Acad Dermatol. 2020;82(3):574]. J Am Acad Dermatol. 2020;82(1):161-201. doi:10.1016/j.jaad.2019.08.049
Paller AS, Siegfried EC, Langley RG, et al; Etanercept Pediatric Psoriasis Study Group. Etanercept treatment for children and adolescents with plaque psoriasis. N Engl J Med. 2008;358(3):241-251. doi:10.1056/NEJMoa066886
Philipp S, Menter A, Nikkels AF, et al. Ustekinumab for the treatment of moderate-to-severe plaque psoriasis in paediatric patients (≥ 6 to < 12 years of age): efficacy, safety, pharmacokinetic and biomarker results from the open-label CADMUS Jr study. Br J Dermatol. 2020;183(4):664-672. doi:10.1111/bjd.19018
Relvas M, Torres T. Pediatric psoriasis. Am J Clin Dermatol. 2017;18(6):797-811. doi:10.1007/s40257-017-0294-9
Sun HY, Phan K, Paller AS, Sebaratnam DF. Biologics for pediatric psoriasis: a systematic review and meta-analysis. Pediatr Dermatol. 2022;39(1):42-48. doi:10.1111/pde.14870
This information is brought to you by Engage Health Media and is not sponsored, endorsed, or accredited by Maui Derm Hawaii 2024.