Dermatology
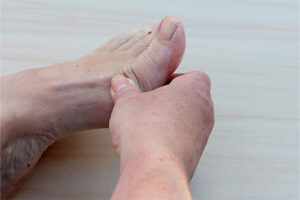
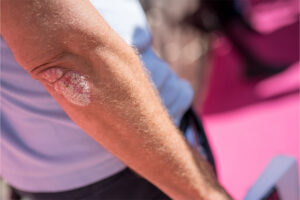
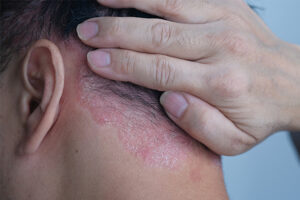
Plaque Psoriasis
COVID-19 Vaccination in Patients With Psoriasis
Overview
At this time, the recommendation is that, in most cases, barring contraindication, patients with psoriatic disease should get the first COVID-19 vaccine that is available to them.
Expert Commentary
Joel M. Gelfand, MD, MSCE
|
|
“Regarding systemic therapies, the Task Force recommendation is that, in most cases, patients should continue their biologic and oral therapies for their psoriatic disease when they are getting vaccinated against COVID-19.”
Regarding COVID-19 vaccination in patients with psoriasis, it is important to keep up with the latest recommendations, given that our understanding of the disease is constantly evolving. The recommendations from the National Psoriasis Foundation (NPF) COVID-19 Task Force are intended as a living document that will be updated as the science evolves. At this time, the Task Force recommends that, in most cases, barring contraindication, patients with psoriatic disease get the first COVID-19 vaccine that is available to them. There are two 2-dose mRNA vaccines and one 1-dose adenovirus-based vaccine. The mRNA vaccines have approximately 95% to 96% protection against symptomatic infection with COVID-19 and are more than 99% protective against severe COVID-19 illness. Currently, virtually all COVID-19–related deaths in the United States occur in unvaccinated patients. Up-to-date recommendations are available from the NPF COVID-19 Resource Center (www.psoriasis.org/covid-19-task-force-guidance-statements).
Regarding systemic therapies, the Task Force recommendation is that, in most cases, patients should continue their biologic and oral therapies for their psoriatic disease when they are getting vaccinated against COVID-19. For patients who are to receive a 1-dose adenovirus-based vaccine, those aged 60 years and older with a major comorbidity for poor COVID-19 outcomes (eg, obesity, diabetes, or chronic obstructive pulmonary disease) and those who are taking methotrexate for their psoriasis may, in consultation with their prescriber, consider holding their methotrexate for 2 weeks after getting the COVID-19 vaccine if their psoriasis is well controlled. The data for this recommendation is indirect and is from a Korean study that showed that patients with rheumatoid arthritis who received a flu shot developed a marginally better vaccine response based on antibody levels if their methotrexate was held for 2 weeks after the shot compared with those who stayed on methotrexate. Shared decision making between the clinician and the patient is recommended to guide discussions about the use of systemic therapies during the pandemic.
Further, out of an abundance of caution, and until more data emerge, the NPF Task Force recommends that patients with psoriatic disease taking abatacept, cyclosporine, leflunomide, glucocorticoids (eg, prednisone), methotrexate, or tofacitinib continue masking and social distancing precautions when they are in contact with people who are not vaccinated against COVID-19 or whose vaccination status is not verifiable.
Neither psoriasis nor its therapies appear to meaningfully alter a patient’s risk of contracting COVID-19 or having a worse course of disease. The data are not perfect, and there is some uncertainty; however, generally speaking, the well-publicized risk factors such as age and major health problems (eg, obesity, cardiovascular disease, and kidney disease) are the main drivers of poor COVID-19 outcomes.
References
Brownstone ND, Thibodeaux QG, Reddy VD, et al. Novel coronavirus disease (COVID-19) and biologic therapy in psoriasis: infection risk and patient counseling in uncertain times. Dermatol Ther (Heidelb). 2020;10(3):1-11. doi:10.1007/s13555-020-00377-9
Chiricozzi A, Gisondi P, Bellinato F, Girolomoni G. Immune response to vaccination in patients with psoriasis treated with systemic therapies. Vaccines (Basel). 2020;8(4):769. doi:10.3390/vaccines8040769
Gelfand JM, Armstrong AW, Bell S, et al. National Psoriasis Foundation COVID-19 Task Force guidance for management of psoriatic disease during the pandemic: version 2 – advances in psoriatic disease management, COVID-19 vaccines, and COVID-19 treatments. J Am Acad Dermatol. 2021;84(5):1254-1268. doi:10.1016/j.jaad.2020.12.058
Lima XT, Cueva MA, Lopes EM, Alora MB. Severe COVID-19 outcomes in patients with psoriasis. J Eur Acad Dermatol Venereol. 2020;34(12):e776-e778. doi:10.1111/jdv.16867
Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029-1072. doi:10.1016/j.jaad.2018.11.057
National Psoriasis Foundation. COVID-19 resource center. Accessed July 19, 2021. https://www.psoriasis.org/covid-19-resource-center/
National Psoriasis Foundation. COVID-19 Task Force guidance statements. Accessed July 19, 2021. https://www.psoriasis.org/covid-19-task-force-guidance-statements/
Park JK, Lee YJ, Shin K, et al. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis. 2018;77(6):898-904. doi:10.1136/annrheumdis-2018-213222