Dermatology
Plaque Psoriasis
Evidence-Based Medicine on Laboratory Monitoring for Biologics
Overview
Our featured experts discuss baseline and routine laboratory monitoring for patients on biologic therapies. Additionally, they consider the prospect of therapeutic drug monitoring (TDM), a practice that is not well established in psoriasis care.
What is your approach to monitoring patients who are on a biologic therapy?
Steven R. Feldman, MD, PhD
|
|
“There is a balance between too much and not enough monitoring, and I think that physicians are in a good position to use their best judgment.”
I understand that gastroenterologists have been doing TDM to determine the blood levels of biologics; however, that has not been a part of my practice in the dermatology world. If the patient is doing well, it would not matter to me if the drug levels were low. Conversely, if the patient is not doing well, I would change the treatment regardless of what the TDM showed.
Generally, I take a minimalist approach to the laboratory monitoring of biologics, and I tend to follow recommendations in the US Food and Drug Administration–approved drug labels. For tumor necrosis factor (TNF) inhibitors, I perform a baseline hepatitis profile, as well as baseline and annual tuberculosis (TB) tests. For other biologics, I order only a baseline TB test, and I tend to repeat those TB tests annually, in part because my nursing staff encourages me to do so; annual TB testing is not required in the label for interleukin-17 (IL-17) and IL-23 inhibitors.
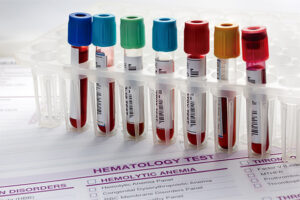
A famous psoriasis specialist once told me that, before starting treatment with any drug that affects the immune system, he obtains a panel of laboratory work, including complete blood count, complete metabolic profile, urinalysis and chest x-ray, antinuclear antibody, C-reactive protein, lipid profile, HIV testing, and hepatitis panel (and repeat at appropriate intervals). He suggested that I do this too because I might find something and because there is no downside. Ironically, however, a downside to this approach is that I probably would find something, such as a false-positive test, that is not relevant and that could lead to further testing and morbidity.
Peter van de Kerkhof, MD, PhD, and colleagues published a study in which they had followed patients on biologic therapy for more than 5 years, and laboratory abnormalities were detected approximately 14% to 26% of the time. However, none of these abnormalities were related to the psoriasis therapy, and they did not have to stop or change the treatment. Thus, doing more laboratory tests may be more trouble than it is worth. There is a balance between too much and not enough monitoring, and I think that physicians are in a good position to use their best judgment.
Joel M. Gelfand, MD, MSCE
|
|
“Regarding TDM in psoriasis, even if we could get drug levels for biologics, the normal therapeutic range is not well defined.”
The 2019 National Psoriasis Foundation–American Association of Dermatology guidelines added some flexibility in their recommendations on monitoring, noting that serious opportunistic infections (eg, TB) are rarely observed in clinical trials or in practice with IL-17/IL-23 inhibitors. For TNF inhibitors and for IL-17/IL-23 inhibitors, yearly testing for latent TB is recommended for patients who are in contact with individuals with active TB because of travel, work, or a family relationship, and for patients with selected underlying medical conditions. For those who are not at high risk, the guidelines suggest that screening should be done at the discretion of the dermatologist.
As Dr Feldman mentioned, follow-up monitoring can be low yield, as these therapies do not really cause changes in the blood counts or in the liver or kidney function tests. You can have idiosyncratic effects on blood counts, and there are rare observations of TNF inhibitors being associated with changes in the liver function tests. So, the way that I approach it with my patients is that when I start a new biologic, I will check lab tests for 3 to 4 months to make sure that there is nothing unusual going on, such as an idiosyncratic reaction.
Regarding TDM in psoriasis, even if we could get drug levels for biologics, the normal therapeutic range is not well defined. As dermatologists, we have the benefit of readily determining whether a biologic is working based on the patient’s skin response; however, in patients with rheumatic disease, it is more challenging to know whether the symptoms are from other etiologies (eg, osteoarthritis), as opposed to the drug not targeting the disease of interest.
Alice B. Gottlieb, MD, PhD
|
|
“If it were possible to obtain the levels of the drugs that we use and to adjust our doses appropriately, I think that that would be fairly important.”
Before starting immunosuppressives, I think that it is a good idea to screen for TB, and I also want to know whether the patient has hepatitis B or C, as that would affect my treatment choice. Further, I want to know whether the patient is leukopenic, so I do get a complete blood count, and I get a metabolic profile because some of these biologic drugs can affect the liver. If I am prescribing teratogenic drugs such as methotrexate, I will get a pregnancy test, where indicated.
In terms of monitoring patients on the treatment, however, I am fairly minimalist. Since almost every package insert recommends screening for TB, my feeling is that, if you screen for TB to begin with, then you should monitor for it on an ongoing basis as well. So, I usually get a TB test once per year. Will I delay treatment if the results are late? No. But that is probably the only monitoring that I do annually, unless there is a clinical reason for me to do so.
Regarding routine TDM, I think that it would be a great idea if it were clinically available because there are many studies showing that biologic drug levels matter. Patients do vary in their levels, for several reasons. If it were possible to obtain the levels of the drugs that we use and to adjust our doses appropriately, I think that that would be fairly important.
References
Hermans C, Herranz P, Segaert S, Gils A. Current practice of therapeutic drug monitoring of biopharmaceuticals in psoriasis patients. Ther Drug Monit. 2017;39(4):356-359. doi:10.1097/ftd.0000000000000401
Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029-1072. doi:10.1016/j.jaad.2018.11.057
Nash P, Kerschbaumer A, Dörner T, et al. Points to consider for the treatment of immune-mediated inflammatory diseases with Janus kinase inhibitors: a consensus statement. Ann Rheum Dis. 2021;80(1):71-87. doi:10.1136/annrheumdis-2020-218398
Papamichael K, Vogelzang EH, Lambert J, Wolbink G, Cheifetz AS. Therapeutic drug monitoring with biologic agents in immune mediated inflammatory diseases. Expert Rev Clin Immunol. 2019;15(8):837-848. doi:10.1080/1744666X.2019.1630273
Schots L, Grine L, Soenen R, Lambert J. Dermatologists on the medical need for therapeutic drug monitoring of biologics in psoriasis: results of a structured survey. J Dermatolog Treat. 2020 Oct 15;1-9. doi:10.1080/09546634.2020.1832649
Singh JA, Guyatt G, Ogdie A, et al. Special article: 2018 American College of Rheumatology/National Psoriasis Foundation guideline for the treatment of psoriatic arthritis. Arthritis Care Res (Hoboken). 2019;71(1):2-29. doi:10.1002/acr.23789
Tsakok T, Wilson N, Dand N, et al; British Association of Dermatologists Biologic and Immunomodulators Register (BADBIR) Study Group and the Psoriasis Stratification to Optimise Relevant Therapy (PSORT) Consortium. Association of serum ustekinumab levels with clinical response in psoriasis. JAMA Dermatol. 2019;155(11):1235-1243. doi:10.1001/jamadermatol.2019.1783
van Lümig PPM, Driessen RJB, Roelofs-Thijssen MAMA, Boezeman JBM, van de Kerkhof PCM, de Jong EMGJ. Relevance of laboratory investigations in monitoring patients with psoriasis on etanercept or adalimumab. Br J Dermatol. 2011;165(2):375-382. doi:10.1111/j.1365-2133.2011.10329.x