Oncology
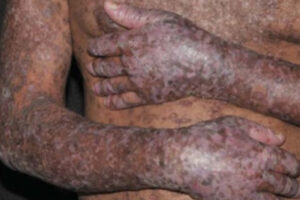
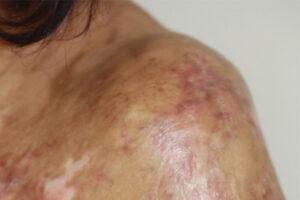
Chronic Graft-versus-Host Disease
Advancements in the Management of Chronic Graft-versus-Host Disease
Overview
As the underlying pathways involved in chronic graft-versus-host disease (cGVHD) are becoming better understood, novel therapies targeting these pathways have emerged. Our featured experts outline these developments and share their thoughts on the future.
Are advancements in the science of cGVHD translating into tangible wins in the clinic?
Corey Cutler,, MD, MPH, FRCPC
|
|
“The answer is a solid yes. As we have deciphered more and more of the important pathways involved in the immunobiology of cGVHD, we have been able to leverage that knowledge to develop novel therapeutics.”
The answer is a solid yes. As we have deciphered more and more of the important pathways involved in the immunobiology of cGVHD, we have been able to leverage that knowledge to develop novel therapeutics. I think that the developing science will lead to even more of these tangible benefits in the future. There is a lot of research going on right now that will ultimately improve outcomes in cGVHD.
There are 3 US Food and Drug Administration (FDA)–approved treatments for cGVHD (ie, ruxolitinib, ibrutinib, and belumosudil). At least 1 additional agent, axatilimab, is currently undergoing a pivotal trial, which may lead to a fourth FDA approval. Despite the currently available and emerging therapies, we lack an understanding of how to identify the pathway that is most relevant for an individual patient. While the majority of patients with cGVHD will respond to a therapy such as ruxolitinib or belumosudil, response rates would improve if we could preselect agents based on the individual patient’s phenotype and the likelihood of response. That is our next step, and it will be a major step forward.
We are also observing advancements in cGVHD prophylaxis. We are trying to determine whether we can prevent cGVHD through interventions at the time of allogeneic hematopoietic stem cell transplantation or shortly thereafter. Newer conditioning regimens and transplantation techniques are associated with lower rates of acute GVHD, which should reduce the number of patients with severe cGVHD. Finally, the science will also help us understand how to design trials of rational treatment combinations for cGVHD. We currently use the available agents sequentially. While targeting multiple pathways simultaneously might improve outcomes, this needs to be shown in studies.
Edmund K. Waller, MD, PhD, FACP
|
|
“We have learned that JAK/STAT signaling and ROCK signaling are important in T-cell–mediated immune responses, and studies have also shown the importance of B-cell receptor signaling in the pathophysiology of cGVHD.”
Over the past decade, we have gained a better understanding of the molecular signals that drive pathogenic T cells in cGVHD. We have learned that JAK/STAT signaling and ROCK signaling are important in T-cell–mediated immune responses, and studies have also shown the importance of B-cell receptor signaling in the pathophysiology of cGVHD.
Ibrutinib was the first agent to be FDA approved for cGVHD. It is a tyrosine kinase inhibitor that targets the BTK pathway in B cells and the ITK pathway in T cells. By interfering with signaling through the B-cell receptor, it is thought that ibrutinib might have a favorable effect on the production of autoreactive antibodies that contribute to fibrosis. However, we do not yet have a clear understanding of which therapeutics might be of particular benefit for specific fibrotic features or types of organ involvement in cGVHD.
Ruxolitinib is a JAK inhibitor that is widely used in patients who have persistent or progressive disease after 1 to 2 prior lines of therapy. The REACH3 study by Zeiser et al showed that the ruxolitinib-treated population had a higher overall response at 6 months—nearly double that of the control population—and the durability of that response was superior to the control arm's standard-of-care treatment.
Belumosudil, another tyrosine kinase inhibitor, targets the ROCK pathway, which is involved in the fibrotic process of cGVHD. Activated macrophages produce growth factors that activate profibrotic mediators such as TGFB. These mediators activate ROCK2, leading to the deposition of collagen bands by fibroblasts. Such deposition can cause the development of fibrotic features of cGVHD, such as limited joint mobility, tear duct obstruction, or pulmonary fibrosis. In the ROCKstar study, patients on belumosudil experienced an improvement in cGVHD across all organ sites, particularly those that were associated with fibrosis, suggesting that belumosudil might be particularly effective in patients with some of the fibrotic features of cGVHD. However, there was no control arm in this study. Thus, it is difficult, once again, to make any definitive claims that patients with certain fibrotic disease manifestations would benefit more from one particular drug than another.
David Miklos, MD, PhD
|
|
“As we target the individual immune components—whether by depleting cells in the graft or by treating the cGVHD—the reconstitution of a competent immune system is going to be a key challenge.”
As previously noted, we currently have 3 novel therapies for cGVHD, and we are making progress with transplant technology. However, whether you consider cGVHD therapies or graft engineering, these interventions are essentially parsing the immune system, and the immune system is complicated.
We need to better assess the impact of cGVHD therapies and engineered grafts on immune reconstitution and the retention of antitumor benefits. As we target the individual immune components—whether by depleting cells in the graft or by treating the cGVHD—the reconstitution of a competent immune system is going to be a key challenge. Early allogeneic hematopoietic stem cell transplantation used unmanipulated whole bone marrow products and reconstituted all cell lineages in a homeostatic manner. Now when parsing the individual components of the immune system by depleting specific cells from grafts or blocking effector pathways, one can expect to observe challenges in immune reconstitution. Growing back immunity is difficult if you do not have all of the parts. It goes beyond simply replacing stem cells. If you replace a stem cell in patient who has been incredibly depleted with radiation, chemotherapy, and other targeted treatments, that individual's ability to reconstitute their immune system will be less robust.
Moreover, there is an increasing recognition of the risk of infection in patients with cGVHD who are being treated with immunosuppressive regimens. It is really T-cell depletion that is leaving the patient at risk for infection. Although these treatments for cGVHD have allowed us to accomplish much more than what we were able to accomplish with steroids alone, combining 2 of them puts the patient at risk for toxicity.
Future advances will rely, in part, on an improved understanding of donor cell behavior after transplantation. The success of chimeric antigen receptor T-cell therapy can be attributed to the direct linkage of the engineered cells to the target (eg, CD19), the expansion of the treatment itself, and the qualitative measurement of efficacy. We need to apply the same rigor in bone marrow transplantation (eg, tracking the donor cells and their interactions with targets) to better understand the complexity of cGVHD.
References
Cutler C, Lee SJ, Arai S, et al. Belumosudil for chronic graft-versus-host disease after 2 or more prior lines of therapy: the ROCKstar study [published correction appears in Blood. 2022;139(11):1772]. Blood. 2021;138(22):2278-2289. doi:10.1182/blood.2021012021
Hamilton BK. Updates in chronic graft-versus-host disease. Hematology Am Soc Hematol Educ Program. 2021;2021(1):648-654. doi:10.1182/hematology.2021000301
Holtzman NG, Pavletic SZ. The clinical landscape of chronic graft-versus-host disease management in 2021. Br J Haematol. 2022;196(4):830-848. doi:10.1111/bjh.17835
Martini DJ, Chen Y-B, DeFilipp Z. Recent FDA approvals in the treatment of graft-versus-host disease. Oncologist. 2022;27(8):685-693. doi:10.1093/oncolo/oyac076
Miklos D, Cutler CS, Arora M, et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood. 2017;130(21):2243-2250. doi:10.1182/blood-2017-07-793786
Miklos D, Zaid MA, Cooney JP, et al. Ibrutinib vs placebo in combination with corticosteroids in patients with new-onset chronic graft-versus-host disease (cGVHD): results from the randomized, double-blind phase 3 INTEGRATE study [abstract S235]. Abstract presented at: European Hematology Association 2021 Virtual Congress; June 9-17, 2021.
Saidu NEB, Bonini C, Dickinson A, et al. New approaches for the treatment of chronic graft-versus-host disease: current status and future directions. Front Immunol. 2020;11:578314. doi:10.3389/fimmu.2020.578314
Sugar A, Hussain M, Chamberlain W, et al. A randomized trial of topical fibrinogen-depleted human platelet lysate treatment of dry eye secondary to chronic graft-versus-host disease. Ophthalmol Sci. 2022;2(3):100176. doi:10.1016/j.xops.2022.100176
Waller EK, Miklos D, Cutler C, et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy: 1-year update of a phase 1b/2 study. Biol Blood Marrow Transplant. 2019;25(10):2002-2007. doi:10.1016/j.bbmt.2019.06.023
Zeiser R, Polverelli N, Ram R, et al; REACH3 Investigators. Ruxolitinib for glucocorticoid-refractory chronic graft-versus-host disease. N Engl J Med. 2021;385(3):228-238. doi:10.1056/NEJMoa2033122