Oncology
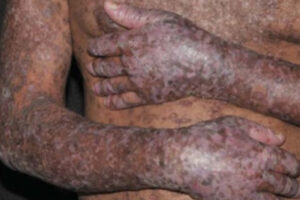
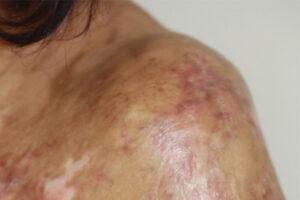
Chronic Graft-versus-Host Disease
Evolving Transplant Technology and Chronic Graft-versus-Host Disease
Overview
Clinicians employ multiple strategies to prevent and treat acute and chronic graft-versus-host disease (aGVHD and cGVHD, respectively). Selective T-cell depletion and graft engineering continue to be explored in an effort to limit the incidence of cGVHD after allogeneic hematopoietic stem cell transplantation.
Expert Commentary
Edmund K. Waller, MD, PhD, FACP
|
|
“ . . . studies are allowing us to dissect the donor cell subsets that are responsible for different functions, such as regulating immune reconstitution and limiting cGVHD, and we are also identifying new molecular targets that could be the subject of therapeutic trials.”
There have been recent advancements in the treatment of cGVHD; however, as with any disease, prevention is preferable to treatment. T cells have a critical role in the pathogenesis of GVHD, and selective T-cell depletion strategies can greatly reduce the incidence of cGVHD. Such strategies aim to reduce alloreactive T cells by manipulating the graft, whether through CD34 selection, the depletion of naive T cells, or the administration of post-transplant cyclophosphamide, which can eliminate alloreactive T cells early after transplantation and before the development of clinical signs of aGVHD or cGVHD.
However, the use of T-cell depletion to prevent cGVHD is associated with certain limitations. T cells are responsible for immune reconstitution after an allogeneic transplant, and immune reconstitution is necessary to both protect patients from opportunistic infections and mediate the graft-vs-leukemia response that is responsible for a significant percentage of the cures that are observed with allogeneic transplants. As such, although T-cell depletion substantially reduces the rates of GVHD, it can also increase the rates of opportunistic infections and relapse.
Ex vivo CD34-selected T-cell depletion is one approach that has been explored. In a study of more than 500 patients with acute leukemia, prophylaxis with CD34+ selection resulted in lower rates of aGVHD and cGVHD compared with antithymocyte globulin–based prophylaxis. Unfortunately, there is a significant delay in immune reconstitution with CD34 selection, along with an increased risk of opportunistic infections. Moreover, the lower rates of moderate and severe cGVHD do not translate to improved survival, as shown in the BMT CTN trial by Luznik et al (NCT02345850).
The most common approach to prophylaxis has probably been the administration of a short pulse of cyclophosphamide after an allogeneic transplant. With this regimen, patients experience some delays in hematopoietic engraftment, but they have remarkably lower levels of cGVHD without compromising the graft-vs-leukemia effect of the transplant.
Nonetheless, there has been great interest in graft engineering, building on the concept of selective depletion, where the goals include preserving the memory compartment of T cells without retaining the naive compartment of T cells that are less differentiated and contain the specificities of alloreactive T cells. For example, Bleakley et al have shown that the depletion of naive T cells from peripheral blood stem cell grafts is associated with a low incidence of severe aGVHD and cGVHD without an apparent excess risk of relapse or nonrelapse mortality in patients with acute leukemia.
We are also becoming more aware of the important contribution of other immune cells in the pathogenesis of cGVHD, particularly regulatory T cells and plasmacytoid dendritic cells. For example, in a preplanned analysis of BMT CTN 0201, we showed that the content of donor plasmacytoid dendritic cells in the marrow of graft recipients was inversely correlated with the incidence of cGVHD after transplantation. In this trial, patients with myelodysplastic syndrome or leukemia were randomized to receive either bone marrow or peripheral blood stem cells from unrelated donors. Those who received a marrow graft with more donor plasmacytoid dendritic cells had a lower incidence of cGVHD than similar patients who received a marrow graft with fewer donor plasmacytoid dendritic cells. Such studies are allowing us to dissect the donor cell subsets that are responsible for different functions, such as regulating immune reconstitution and limiting cGVHD, and we are also identifying new molecular targets that could be the subject of therapeutic trials.
References
Barba P, Hilden P, Devlin SM, et al. Ex vivo CD34+-selected T cell–depleted peripheral blood stem cell grafts for allogeneic hematopoietic stem cell transplantation in acute leukemia and myelodysplastic syndrome is associated with low incidence of acute and chronic graft-versus-host disease and high treatment response. Biol Blood Marrow Transplant. 2017;23(3):452-458. doi:10.1016/j.bbmt.2016.12.633
Biernacki MA, Sheth VS, Bleakley M. T cell optimization for graft-versus-leukemia responses. JCI Insight. 2020;5(9):e134939. doi:10.1172/jci.insight.134939
Bleakley M, Sehgal A, Seropian S, et al. Naive T-cell depletion to prevent chronic graft-versus-host disease. J Clin Oncol. 2022;40(11):1174-1185. doi:10.1200/JCO.21.01755
ClinicalTrials.gov. Calcineurin inhibitor-free interventions BMT CTN 1301 for prevention of graft-versus-host disease (BMT CTN 1301). Updated December 21, 2021. Accessed August 12, 2022. https://clinicaltrials.gov/ct2/show/NCT02345850
Fabrizio VA, Kernan NA, Boulad F, et al. Low toxicity and favorable overall survival in relapsed/refractory B-ALL following CAR T cells and CD34-selected T-cell depleted allogeneic hematopoietic cell transplant. Bone Marrow Transplant. 2020;55(11):2160-2169. doi:10.1038/s41409-020-0926-1
Gooptu M, Romee R, St Martin A, et al. HLA-haploidentical vs matched unrelated donor transplants with posttransplant cyclophosphamide-based prophylaxis. Blood. 2021;138(3):273-282. doi:10.1182/blood.2021011281
Kasamon YL, Ambinder RF, Fuchs EJ, et al. Prospective study of nonmyeloablative, HLA-mismatched unrelated BMT with high-dose posttransplantation cyclophosphamide. Blood Adv. 2017;1(4):288-292. doi:10.1182/bloodadvances.2016002766
Luznik L, O'Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641-650. doi:10.1016/j.bbmt.2008.03.005
Luznik L, Pasquini MC, Logan B, et al. Randomized phase III BMT CTN trial of calcineurin inhibitor–free chronic graft-versus-host disease interventions in myeloablative hematopoietic cell transplantation for hematologic malignancies. J Clin Oncol. 2022;40(4):356-368. doi:10.1200/JCO.21.02293
Scordo M, Hsu M, Jakubowski AA, et al. Immune cytopenias after ex vivo CD34+–selected allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2019;25(6):1136-1141. doi:10.1016/j.bbmt.2018.12.842
van den Brink MRM, Velardi E, Perales M-A. Immune reconstitution following stem cell transplantation. Hematology Am Soc Hematol Educ Program. 2015;2015:215-219. doi:10.1182/asheducation-2015.1.215
Waller EK, Logan BR, Harris WAC, et al. Improved survival after transplantation of more donor plasmacytoid dendritic or naïve T cells from unrelated-donor marrow grafts: results from BMTCTN 0201. J Clin Oncol. 2014;32(22):2365-2372. doi:10.1200/JCO.2013.54.4577
Zhu J, Wang Y, Li J-X, et al. Donor plasmacytoid dendritic cells limit graft-versus-host disease through vasoactive intestinal polypeptide expression. Blood. 2022 Apr 20;blood.2021012561. doi:10.1182/blood.2021012561