Oncology
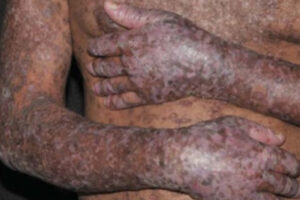
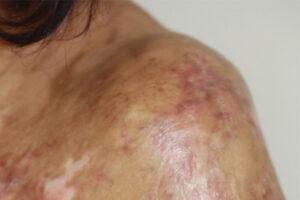
Chronic Graft-versus-Host Disease
Incorporating Novel and Conventional Agents in Chronic Graft-versus-Host Disease
Overview
The treatment of chronic graft-versus-host disease (cGVHD) now includes novel systemic therapies in addition to established agents such as corticosteroids. Our featured experts share their thoughts on the current treatment paradigm.
How do you approach the selection and sequencing of systemic therapies for cGVHD? Is there a role for combination therapy?
Edmund K. Waller, MD, PhD, FACP
|
|
“When we see patients with some of the more severe manifestations of the disease, we feel compelled to do something, but we also want to avoid overtreatment.”
When we see patients with some of the more severe manifestations of the disease, we feel compelled to do something, but we also want to avoid overtreatment. In my opinion, adding another tyrosine kinase inhibitor (TKI) to the treatment regimen for a patient who is already receiving multiple medications (eg, steroids, mycophenolate, a calcineurin inhibitor, and ruxolitinib) is likely to cause more harm than good. If a drug is not effective after an appropriate trial period, I would prefer to replace it with an agent that might be more effective for that individual patient. Polypharmacy is common in the field, but it is not always appropriate.
Steroids remain the mainstay of therapy for most patients with cGVHD, and, even when additional drug therapy is warranted, we usually do not stop the steroids altogether. Rather, the goal of treatment is to obtain some ongoing response and then attempt to taper the steroid to the lowest possible dose. In my practice, I typically treat patients with a steroid first, I add a TKI if they do not respond to the steroid, and then, once the response is established, I begin to slowly taper the steroid over a period of months, followed by a tapering of the TKI.
There is limited evidence to guide the choice of second-line therapy, but we can consider an agent's toxicity profile when deciding what to avoid. For example, ibrutinib should be avoided in a patient with cardiac arrhythmias, bleeding, or bruising, and ruxolitinib should be avoided in a patient with cytopenias. There is support for all of the available drugs across the different clinical manifestations of cGVHD; however, the extent of a particular drug's benefits over another for a particular presentation is not entirely clear.
David Miklos, MD, PhD
|
|
“Regarding the sequencing of therapies, I also think about where the choke points or the strategic opportunities are in view of the timeline of cGVHD pathophysiology.”
I agree with Dr Waller in that, when we consider treatments in combination, the number one consideration is to avoid the potential for toxicity. T-cell depletion leaves the patient at risk for infection.
Regarding the sequencing of therapies, I also think about where the choke points or the strategic opportunities are in view of the timeline of cGVHD pathophysiology. For example, after allogeneic transplants, the rapid growth and expansion of T cells and B cells within lymph nodes in patients with cGVHD occur most commonly at 6 to 12 months post transplant, whereas sclerosis and fibrosis typically occur subsequent to and downstream of that. Thus, we might use inhibitors of T follicular helper cells and allogeneic B cells earlier (ie, during the onset of cGVHD)—possibly with ruxolitinib, which dials down T cells in general. In more established cGVHD, we could shift to managing the consequences of T helper 17 cells, allogeneic antibodies, fibrosis, and activated macrophages, and that is where belumosudil makes sense to me.
The challenge going forward will be to design clinical trials that better assess the impact of these treatments. We will need to measure outcomes that go beyond clinical improvement to make progress. Recently, a randomized placebo-controlled study of ibrutinib vs placebo for new-onset cGVHD failed to show an improved 1-year complete response rate. In that trial, the steroid taper was recommended to maximize the benefits of the steroid. Could we have observed a steroid-sparing benefit by tapering the steroids more rapidly? Perhaps. It has been said that our greatest tool in the treatment of cancer has never been a drug; rather, it is the randomized placebo-controlled trial. We should have placebo-blinded studies, and the blinding should be maintained for longer than 12 months. If we want to determine the steroid-sparing benefits of newer agents, then we should incorporate differing steroid tapering schedules in the trial design.
Corey Cutler,, MD, MPH, FRCPC
|
|
“If an individual has only received steroids, then we are limited to either ruxolitinib or ibrutinib. The choice between ruxolitinib and ibrutinib is often dependent on underlying patient characteristics or the agent's adverse-event profile.”
Regarding the sequencing of agents, ruxolitinib and ibrutinib have relatively similar US Food and Drug Administration labels in the steroid-refractory setting, whereas belumosudil has a label of steroid-refractory disease after 2 or more lines of therapy. Thus, sequencing is often influenced by how many lines of therapy the patient has had. If an individual has only received steroids, then we are limited to either ruxolitinib or ibrutinib. The choice between ruxolitinib and ibrutinib is often dependent on underlying patient characteristics or the agent's adverse-event profile.
The disease that is necessitating the transplant may also come into play. If the patient underwent a transplant for a B-cell malignancy such as chronic lymphocytic leukemia, we might choose a B-cell–active agent such as ibrutinib. For someone who had myelofibrosis or a Janus kinase–driven malignancy, one might consider a Janus kinase inhibitor preferentially. For a patient who has a heavy sclerotic feature to their disease, particularly when they have pulmonary involvement, I tend to choose belumosudil because of its antifibrotic activity.
The treatment of patients with an incomplete response on initial therapy can be challenging. For these individuals, we may consider additive therapy. While combination therapy has not been well studied, it can be difficult to discontinue a drug if it produced a partial response. However, when using the newer agents in patients with steroid resistance, there does not appear to be a great benefit of continuing corticosteroids at high doses. Thus, we have learned to taper steroids to the minimum effective dose.
Finally, we cannot forget that we do have other modalities and drugs that do not have the traditional toxicities of the other agents and can be very effective (eg, mammalian target of rapamycin inhibitors, interleukin 2, and extracorporeal phototherapy).
References
Cutler C, Lee SJ, Arai S, et al. Belumosudil for chronic graft-versus-host disease after 2 or more prior lines of therapy: the ROCKstar study [published correction appears in Blood. 2022;139(11):1772]. Blood. 2021;138(22):2278-2289. doi:10.1182/blood.2021012021
Flowers MED. Progress in the management of chronic GVHD insights into novel therapies to treat and manage GVHD lasting longer than 12 months. Best Pract Res Clin Haematol. 2021;34(1):101253. doi:10.1016/j.beha.2021.101253
Hamilton BK. Updates in chronic graft-versus-host disease. Hematology Am Soc Hematol Educ Program. 2021;2021(1):648-654. doi:10.1182/hematology.2021000301
Miklos D, Cutler CS, Arora M, et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood. 2017;130(21):2243-2250. doi:10.1182/blood-2017-07-793786
Miklos D, Zaid MA, Cooney JP, et al. Ibrutinib vs placebo in combination with corticosteroids in patients with new-onset chronic graft-versus-host disease (CGVHD): results from the randomized, double-blind phase 3 INTEGRATE study [abstract S235]. Abstract presented at: European Hematology Association 2021 Virtual Congress; June 9-17, 2021.
Penack O, Marchetti M, Ruutu T, et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2020;7(2):e157-e167. doi:10.1016/S2352-3026(19)30256-X
Wolff D, Fatobene G, Rocha V, Kröger N, Flowers ME. Steroid-refractory chronic graft-versus-host disease: treatment options and patient management. Bone Marrow Transplant. 2021;56(9):2079-2087. doi:10.1038/s41409-021-01389-5
Wolff D, Hilgendorf I, Wagner-Drouet E, et al. Changes in immunosuppressive treatment of chronic graft-versus-host disease: comparison of 2 surveys within allogeneic hematopoietic stem cell transplant centers in Germany, Austria, and Switzerland. Biol Blood Marrow Transplant. 2019;25(7):1450-1455. doi:10.1016/j.bbmt.2019.03.003
Zeiser R, Polverelli N, Ram R, et al. Ruxolitinib (RUX) vs best available therapy (BAT) in patients (pts) with glucocorticoid-refractory chronic graft-vs-host disease (cGVHD): primary findings from the phase 3, randomized REACH3 study [abstract 82]. Abstract presented at: The 2021 Transplantation & Cellular Therapy Meetings of the American Society for Transplantation and Cellular Therapy and the Center for International Blood & Marrow Transplant Research; February 8-12, 2021.