Dermatology
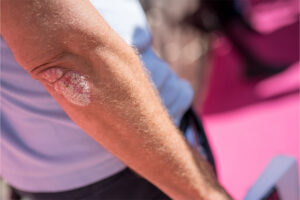
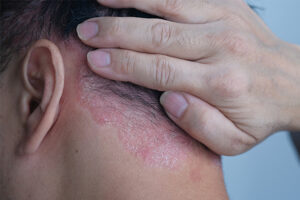
Plaque Psoriasis
Management of Plaque Psoriasis With Nail Involvement
Overview
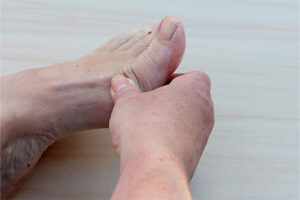
Nail involvement is common in patients with plaque psoriasis. Our featured experts review the differing presentations of nail psoriasis and the factors that influence treatment decisions.
What factors do you consider when treating patients who have plaque psoriasis with nail involvement?
Boni E. Elewski, MD
|
|
“ . . . when I see a patient with severe nail psoriasis, I consider the high likelihood that they also have psoriatic arthritis or are at risk for its development.”
Only approximately 5% to 6% of patients with psoriasis have nail-only disease; however, perhaps 50% of those with psoriasis and up to 80% to 90% of those with psoriatic arthritis are affected by nail lesions. Nail involvement is correlated with more severe disease, earlier disease onset, and a higher risk of psoriatic arthritis. In fact, when I see a patient with severe nail psoriasis, I consider the high likelihood that they also have psoriatic arthritis or are at risk for its development. Often, patients do not link joint pain to skin disease, so I think that it is important to ask them about and to examine their joints (eg, their distal interphalangeal joints).
Consensus recommendations by Rigopoulos et al included a practical approach to more limited presentations based on the number of nails affected, also highlighting the importance of the lesion location (eg, nail bed vs matrix). When treating nail-only psoriasis affecting 3 or fewer nails in the matrix area only, there was a strong consensus that intralesional steroid injections are the first-line treatment of choice. The group did not endorse a definite threshold for initiating systemic therapy for cases of nail psoriasis in the setting of minimal skin disease but did state the importance of considering the patients’ needs and the impact that nail psoriasis has on their quality of life when making decisions about therapy.
Disease-related quality of life can be really horrible in patients with bad nail disease—especially among men, in my experience, because they usually do not want to hide it with nail polish. I do use systemic treatments such as the interleukin (IL)-17 and IL-23 inhibitors for nail psoriasis, and they have very good data on sustained efficacy. There are also oral options for systemic therapy, such as apremilast. Deucravacitinib, a more recent addition, is another oral option. Oral treatments can be perceived by individuals with nail psoriasis as being more patient friendly.
Apremilast is approved by the US Food and Drug Administration for adults with active psoriatic arthritis, as well as for those with mild to moderate plaque psoriasis, who are candidates for systemic therapy. It works reasonably well in psoriatic arthritis, and it is among my first choices for a patient with mild plaque psoriasis (eg, those with approximately 1% body surface area affected, some nail psoriasis, or some scalp psoriasis). Apremilast may be a good starting point, but if I am choosing among the biologics and also looking for efficacy in psoriatic arthritis, adalimumab could be considered, as well as IL-17 blockers, which work fast and are very effective for both nail and skin disease.
Steven R. Feldman, MD, PhD
|
|
“Importantly, nails grow slowly, and the physical findings of nail psoriasis tend to be reflective of disease activity that occurred months earlier. Patients with nail psoriasis must be very persistent for treatment to be effective. It takes a while after treatment initiation before they start seeing an improvement in symptoms, and it takes even longer for the nail to clear.”
Characteristic features of nail psoriasis include irregular pitting, onycholysis with or without a red border, and oil spots. Other clinical features such as splinter hemorrhages and subungual hyperkeratosis may also be observed. Patients with nail psoriasis may have dents in their nails, as well as nail discoloration, thickening, or crumbling. Further, they may experience pain, functional impairment, and social stigma.
Importantly, nails grow slowly, and the physical findings of nail psoriasis tend to be reflective of disease activity that occurred months earlier. Patients with nail psoriasis must be very persistent for treatment to be effective. It takes a while after treatment initiation before they start seeing an improvement in symptoms, and it takes even longer for the nail to clear. This delay in response is a major hurdle when it comes to treatment adherence among individuals with nail psoriasis.
Topical therapy is difficult because, as Dr Elewski alluded to, a topical agent is not going to be helpful in a patient with nail pitting only because that disease is in the matrix. For someone who is experiencing onycholysis and subungual hyperkeratosis, a topical agent might be helpful if it can be applied under the nail. Thus, it is important to know where the disease is in the nail when deciding whether a topical agent would be effective.
Finally, when considering biologics in patients with nail psoriasis, the timing of treatment response may differ among each of the agents in this class. An agent with a very fast onset of action (an IL-17 antagonist) might produce better results at an earlier time point, while other agents might show similar efficacy at a later time point.
Bruce E. Strober, MD, PhD
|
|
“I would emphasize that it is entirely legitimate to treat significant psoriatic nail disease with systemic agents, even in the absence of significant skin disease.”
I would emphasize that it is entirely legitimate to treat significant psoriatic nail disease with systemic agents, even in the absence of significant skin disease. Nail psoriasis is one of those special areas of involvement. Additionally, clinical trial data in nail psoriasis indicate that assessments really need to be done at 6 months or even later after treatment initiation to evaluate efficacy. In fact, I do not generally even consider nail outcomes data if they are based on a 12-week efficacy metric—you need to go out 6 months or longer.
Generally, it might be true that biologics that work better for the skin are also likely to work better for the nails. I gravitate toward the IL-17 inhibitors, which are very effective in treating psoriatic nail disease. In fact, the IL-17 inhibitor ixekizumab probably has some of the most impressive data. That is not to say that the IL-23 drugs do not work on the nails; they have data that show positive efficacy as well.
Interestingly, Foley et al conducted a secondary analysis of the VOYAGE 1 and VOYAGE 2 trials, examining responses to adalimumab vs guselkumab at different sites. Guselkumab was associated with significant improvements over adalimumab in psoriasis affecting the scalp, palms, and/or soles, but improvements in the fingernails were similar between the 2 drugs. These results seem to suggest that it is possible to have a disconnect between skin and nails regarding treatment efficacy. Further, I think that adalimumab might have efficacy in psoriatic arthritis that extends to nail psoriasis. That is, we often see distal interphalangeal inflammation in psoriatic arthritis, and, if adalimumab is reducing that inflammation, that may also improve nail outcomes.
Finally, deucravacitinib is a newly approved oral tyrosine kinase 2 inhibitor, and some data suggest that it may be effective for nail psoriasis. In the long run, I would probably be more likely to use deucravacitinib as an oral agent for psoriatic nail disease than apremilast, as I believe that it will have better efficacy with this special area.
References
Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2022 Jul 9;S0190-9622(22)02256-3. doi:10.1016/j.jaad.2022.07.002
Blauvelt A, Papp KA, Griffiths CEM, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405-417. doi:10.1016/j.jaad.2016.11.041
Coates LC, Soriano ER, Corp N, et al; GRAPPA Treatment Recommendations Domain Subcommittees. Group for research and assessment of psoriasis and psoriatic arthritis (GRAPPA): updated treatment recommendations for psoriatic arthritis 2021 [published correction appears in Nat Rev Rheumatol. 2022 Oct 10. doi:10.1038/s41584-022-00861-w]. Nat Rev Rheumatol. 2022;18(8):465-479. doi:10.1038/s41584-022-00798-0
Foley P, Gordon K, Griffiths CEM, et al. Efficacy of guselkumab compared with adalimumab and placebo for psoriasis in specific body regions: a secondary analysis of 2 randomized clinical trials. JAMA Dermatol. 2018;154(6):676-683. doi:10.1001/jamadermatol.2018.0793
Kaeley GS, Eder L, Aydin SZ, Rich P, Bakewell CJ. Nail psoriasis: diagnosis, assessment, treatment options, and unmet clinical needs. J Rheumatol. 2021;48(8):1208-1220. doi:10.3899/jrheum.201471
Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76(3):418-431. doi:10.1016/j.jaad.2016.11.042
Reich K, Conrad C, Kristensen LE, et al. Network meta-analysis comparing the efficacy of biologic treatments for achieving complete resolution of nail psoriasis. J Dermatolog Treat. 2022;33(3):1652-1660. doi:10.1080/09546634.2021.1892024
Rigopoulos D, Baran R, Chiheb S, et al. Recommendations for the definition, evaluation, and treatment of nail psoriasis in adult patients with no or mild skin psoriasis: a dermatologist and nail expert group consensus. J Am Acad Dermatol. 2019;81(1):228-240. doi:10.1016/j.jaad.2019.01.072
Rusk AM, Fleischer AB Jr. In psoriasis treatment, greater improvement in skin severity predicts greater improvement in nail severity. J Dermatolog Treat. 2021;32(8):894-897. doi:10.1080/09546634.2020.1720578
Stein Gold L, Papp K, Pariser D, et al. Efficacy and safety of apremilast in patients with mild-to-moderate plaque psoriasis: results of a phase 3, multicenter, randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2022;86(1):77-85. doi:10.1016/j.jaad.2021.07.040
Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 POETYK PSO-2 trial. J Am Acad Dermatol. 2022 Sep 14;S0190-9622(22)02643-3. doi:10.1016/j.jaad.2022.08.061
Tillett W, Egeberg A, Sonkoly E, et al. POS1033: dynamics of nail psoriasis with guselkumab treatment and withdrawal in association with skin response and patient-reported outcomes: a post hoc analysis of the VOYAGE2 phase 3 trial. Ann Rheum Dis. 2022;81:830. doi:10.1136/annrheumdis-2022-eular.1478