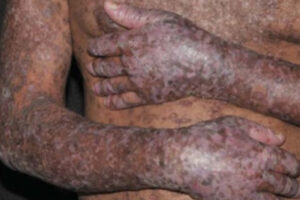
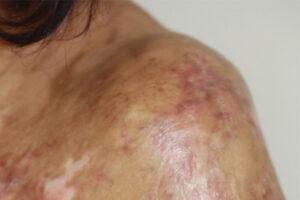
Oncology
Chronic Graft-versus-Host Disease
Chronic Graft-versus-Host Disease: Management and Monitoring Recommendations
Overview
Most patients with chronic graft-versus-host disease (cGVHD) do not achieve a durable resolution of disease activity with initial treatment. The use of comprehensive clinical assessment and tracking tools, such as those developed the National Institutes of Health (NIH), can aid in the identification of patients with cGVHD who may benefit from additional therapeutic intervention.
What are the current tools for assessing and monitoring cGVHD?
Corey Cutler,, MD, MPH, FRCPC
|
|
“The assessment and follow-up tools developed by the NIH are very helpful, and we should be using them, even in patients who are not enrolled in clinical trials.”
The assessment and follow-up tools developed by the NIH are very helpful, and we should be using them, even in patients who are not enrolled in clinical trials. In the future, there will be new topics to learn about in this field that we had not previously considered, as well as new questions that will require answers. Eventually, we will need to mine our databases, and the only way we can do that effectively is by performing the NIH assessments in all of our patients. Some of the assessments may be time consuming, but integration into the electronic medical records system has made it somewhat easier in many cases.
It is exciting that there are several drugs that are being actively studied and developed for the treatment of cGVHD. In addition to ibrutinib, which was approved by the US Food and Drug Administration for cGVHD in 2017, we have seen the recent approvals of 2 new agents (ie, belumosudil and ruxolitinib), and there will likely be other approvals to follow. Ibrutinib, belumosudil, and ruxolitinib are indicated for use after the failure of prior lines of therapy in adults and pediatric patients aged 12 years and older with cGVHD. We will have to learn how to sequence these therapies and use them in various combinations.
I think that the field is really beginning to grow. From the perspective of ongoing monitoring, comprehensive assessments are important because they will provide data that can be analyzed to address new questions as they emerge.
Daniel R. Couriel, MD, MS
|
|
“In terms of the development of useful biomarkers, we are still in the early stages. I do not foresee that we will stop relying on the clinical manifestations of cGVHD for patient follow-up anytime soon.”
I think that we can all agree that most clinicians currently primarily rely on the clinical manifestations of cGVHD to assess and monitor these patients. The assessment and follow-up tools developed by the NIH are useful, and we do, indeed, use them to follow up with patients, clinically. In terms of the development of useful biomarkers, we are still in the early stages. I do not foresee that we will stop relying on the clinical manifestations of cGVHD for patient follow-up anytime soon.
One of the challenges with the NIH consensus recommendations is that the workup can entail an assessment that is quite involved, at least initially, so we really need to incorporate the NIH recommendations into our regular workflow. We like to incorporate at least the documentation portion of the NIH assessment scores into our electronic medical records so that, eventually, the task becomes simpler.
Regarding therapies for cGVHD, as Dr Cutler noted, we now have ibrutinib, belumosudil, and ruxolitinib, so we can choose from among these therapies based on clinical experience and preferences. There is a lot of excitement about selective Rho-associated coiled-coil–containing protein kinase 2 (ROCK2) inhibition with belumosudil. This is an agent that appears to work on both the inflammation and fibrosis of cGVHD. Ibrutinib was approved based on a phase 1b/2 study, and I think that we need a lot more real-life experience with ibrutinib. I agree that we will have to learn how to sequence these therapies.
And then, there are agents that are earlier in their clinical development, such as axatilimab, an experimental monoclonal antibody that acts on colony stimulating factor 1 receptor, which is believed to regulate monocyte and macrophage survival and functioning.
Sergio A. Giralt, MD
|
|
“Another benefit of the consensus NIH assessment is that it can be used as a training tool to help physicians and advanced practice providers develop expertise; this is a disease that really requires expertise to me managed effectively.”
The NIH consensus assessments drive good patient care, in part because they are comprehensive. Additionally, the NIH tools, in some ways, serve to highlight that cGVHD is a disease that requires a multidisciplinary approach. Dr Couriel is one of the pioneers in the development of the multidisciplinary cGVHD clinic. The NIH has perhaps the largest and the most comprehensive cGVHD clinic in operation today, and we have started to develop one at the Memorial Sloan Kettering Cancer Center.
Even if a multidisciplinary cGVHD clinic is only operating through a consultative model, it can still have a lot of value. When a patient is seen at such a clinic, they receive a comprehensive assessment, and that information is shared with the initiating clinician. More limited assessments can be done for short-term follow-ups.
Another benefit of the NIH consensus assessments is that they can be used as a training tool to help physicians and advanced practice providers develop expertise; this is a disease that really requires expertise to be managed effectively.
References
ClinicalTrials.gov. A study of axatilimab at 3 different doses in patients with chronic graft versus host disease (cGVHD) (AGAVE-201). Updated October 29, 2021. Accessed November 5, 2021. https://clinicaltrials.gov/ct2/show/NCT04710576
Cutler CS, Lee SJ, Arai S, et al. Belumosudil for chronic graft-versus-host disease (cGVHD) after 2 or more prior lines of therapy: the ROCKstar study. Blood. 2021 Jul 15;blood.2021012021. doi:10.1182/blood.2021012021
Lee SJ, Wolff D, Kitko C, et al. Measuring therapeutic response in chronic graft-versus-host disease. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. The 2014 Response Criteria Working Group report. Biol Blood Marrow Transplant. 2015;21(6):984-999. doi:10.1016/j.bbmt.2015.02.025
Martin PJ, Lee SJ, Przepiorka D, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: VI. The 2014 Clinical Trial Design Working Group report. Biol Blood Marrow Transplant. 2015;21(8):1343-1359. doi:10.1016/j.bbmt.2015.05.004
Sarantopoulos S, Cardones AR, Sullivan KM. How I treat refractory chronic graft-versus-host disease. Blood. 2019;133(11):1191-1200. doi:10.1182/blood-2018-04-785899
Waller EK, Miklos D, Cutler C, et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy: 1-year update of a phase 1b/2 study. Biol Blood Marrow Transplant. 2019;25(10):2002-2007. doi:10.1016/j.bbmt.2019.06.023
Zanin-Zhorov A, Weiss JM, Nyuydzefe MS, et al. Selective oral ROCK2 inhibitor down-regulates IL-21 and IL-17 secretion in human T cells via STAT3-dependent mechanism. Proc Natl Acad Sci U S A. 2014;111(47):16814-16819. doi:10.1073/pnas.1414189111
Zeiser R, Polverelli N, Ram R, et al; REACH3 Investigators. Ruxolitinib for glucocorticoid-refractory chronic graft-versus-host disease. N Engl J Med. 2021;385(3):228-238. doi:10.1056/NEJMoa2033122