Oncology
Chronic Graft-versus-Host Disease
Chronic Graft-versus-Host Disease: Updates on Treatment and Prevention
Overview
A major recent development in chronic graft-versus-host disease (cGVHD) has been the emergence of novel treatments that more precisely target the disease and its downstream effects. Additionally, researchers continue to pursue more effective interventions at the time of allogeneic hematopoietic stem cell transplantation to prevent GVHD.
What are the recent developments in the treatment and prevention of cGVHD?
Corey Cutler,, MD, MPH, FRCPC
|
|
“The critical development in cGVHD treatment, as opposed to its prevention, is the fact that we are currently using much more targeted therapeutics for cGVHD that might not be influencing the fundamental immunology of alloantigen recognition.”
The critical development in cGVHD treatment, as opposed to its prevention, is the fact that we are currently using much more targeted therapeutics for cGVHD that might not be influencing the fundamental immunology of alloantigen recognition. These agents may be able to treat some select downstream effects of cGVHD (eg, the downregulation of regulatory T cells and the development fibrosis) without interfering with the graft-vs-leukemia effects. I believe that we are currently taking a nice step forward.
In addition to ibrutinib, we now have belumosudil and ruxolitinib for cGVHD, both of which were recently approved by the US Food and Drug Administration for use after the failure of systemic therapy. Belumosudil is an oral selective Rho-associated coiled-coil–containing protein kinase 2 (ROCK2) inhibitor that was approved based on the results of the ROCKstar study. ROCK2 inhibition is known to restore immune homeostasis and shift the Th17/regulatory T cell balance toward regulatory T cells. Belumosudil has also demonstrated a significant reduction of fibrosis in animal models, consistent with the central role of ROCK2 in facilitating multiple fibrotic pathways. Ruxolitinib, an oral selective inhibitor of Janus kinase 1 (JAK1) JAK1 and JAK2 that has shown efficacy among patients with glucocorticoid-refractory or -dependent cGVHD, was approved based on the REACH3 trial.
Further, colony stimulating factor 1 receptor (CSF1R)–dependent monocytes and macrophages are believed to contribute to organ fibrosis, and CSF1R antagonists are currently in development for cGVHD. These agents may help prevent relapse, and, in targeting this specific role in fibrosis, there should not be much of an impact on antitumor immunology. There is currently a phase 2 study in progress that is evaluating the efficacy, safety, and tolerability of axatilimab, an anti-CSF1R monoclonal antibody, at 3 different dose levels in patients with recurrent or refractory active cGVHD who have received at least 2 prior lines of systemic therapy (NCT04710576).
Sergio A. Giralt, MD
|
|
“In the coming years, we hope that calcineurin inhibitors plus methotrexate will no longer be the standard GVHD prevention strategy.”
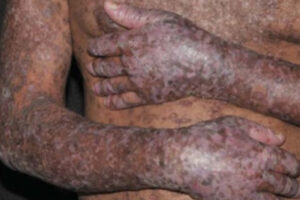
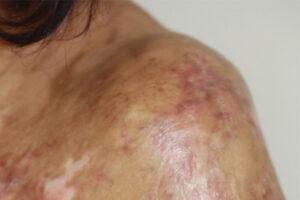
Calcineurin inhibitors plus methotrexate has been the standard prevention strategy for GVHD for the past 30 years. Although this approach has allowed us to perform transplantations more safely and effectively, cGVHD still occurs in approximately 25% of patients receiving bone marrow transplantations and in approximately 50% to 60% of those receiving peripheral blood stem cells. With peripheral blood stem cells being increasingly used, it is unfortunate that cGVHD is somewhat of a signature complication for many post-transplant patients. These individuals might have vitiligo, they might have to wear sunglasses even in dark rooms because of photophobia, or you may notice that they have already developed flexion contractures.
In the coming years, we hope that calcineurin inhibitors plus methotrexate will no longer be the standard GVHD prevention strategy. Fortunately, there has been some progress over the last 10 years. First, post-transplant cyclophosphamide became established as a standard approach to GVHD prevention in the mismatched setting. Additionally, there has been some success with CD34 selection, which has been used for many years now. Donor T cells are critical for the graft-vs-tumor effect, but they carry the risk of GVHD. So, in some settings, CD34 selection may be used to deplete alloreactive donor T cells from the peripheral blood graft and to reduce the rates of GVHD. Now, it remains to be seen whether this approach can be optimized or made to be more user friendly so that it can be used outside of very large and specialized transplant centers.
Newer drugs are also being explored for GVHD prevention, such as abatacept. In the phase 2 ABA2 trial, adding abatacept to unrelated donor hematopoietic stem cell transplantation was safe and appeared promising.
Daniel R. Couriel, MD, MS
|
|
“Another development over the last decade or so is the growing recognition of the value of multispecialty care in the treatment of cGVHD.”
Drs Cutler and Giralt nicely summarized the current landscape. I would add that antithymocyte globulin (ATG) has 2 roles in transplant recipients. When given before transplant, it eliminates host T cells that might reject the graft. However, since ATG has a very long half-life, it will still be present after the transplant, so it will help prevent GVHD.
But there are challenges with ATG that relate to the ideal exposure to ATG for an individual and the unpredictable pharmacokinetics of ATG. When given as a fixed weight-based dose, it is possible that patients could have too much exposure to ATG post transplant, which slows down immune recovery and predisposes one to infection. This could potentially account for some of the observations made in clinical trials of ATG, where the rates of GVHD were reduced but the overall outcomes were not improved.
Another development over the last decade or so is the growing recognition of the value of multispecialty care in the treatment of cGVHD. I have been involved in this collaborative model of care for some time, having started my career at The University of Texas MD Anderson Cancer Center, followed by the University of Michigan, and now here at the University of Utah. I am a firm believer that this disease, because of its complexity and its rarity, is best treated in a dedicated multispecialty care clinic. You need an environment that has a lot of experience with these patients. That environment includes not only you but also an ophthalmologist, a dermatologist, a pulmonologist, a gastroenterologist, and a gynecologist, among others—all of whom need to be experienced with treating these patients because they have a very particular set of needs.
References
Alexander KA, Flynn R, Lineburg KE, et al. CSF-1–dependant donor-derived macrophages mediate chronic graft-versus-host disease. J Clin Invest. 2014;124(10):4266-4280. doi:10.1172/JCI75935
ClinicalTrials.gov. A study of axatilimab at 3 different doses in patients with chronic graft versus host disease (cGVHD) (AGAVE-201). Updated October 29, 2021. Accessed October 11, 2021. https://clinicaltrials.gov/ct2/show/NCT04710576
Cutler CS, Lee SJ, Arai S, et al. Belumosudil for chronic graft-versus-host disease (cGVHD) after 2 or more prior lines of therapy: the ROCKstar study. Blood. 2021 Jul 15;blood.2021012021. doi:10.1182/blood.2021012021
Jagasia M, Lazaryan A, Bachier CR, et al. ROCK2 inhibition with belumosudil (KD025) for the treatment of chronic graft-versus-host disease. J Clin Oncol. 2021;39(17):1888-1898. doi:10.1200/JCO.20.02754
MacDonald KPA., Hill GR, Blazar BR. Chronic graft-versus-host disease: biological insights from preclinical and clinical studies. Blood. 2017;129(1):13-21. doi:10.1182/blood-2016-06-686618
Mehta RS, Holtan SG, Wang T, et al. Composite GRFS and CRFS outcomes after adult alternative donor HCT. J Clin Oncol. 2020;38(18):2062-2076. doi:10.1200/JCO.19.00396
Miklos D, Cutler CS, Arora M, et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood. 2017;130(21):2243-2250. doi:https://doi.org/10.1182/blood-2017-07-793786
Sarantopoulos S, Cardones AR, Sullivan KM. How I treat refractory chronic graft-versus-host disease. Blood. 2019;133(11):1191-1200. doi:10.1182/blood-2018-04-785899
Watkins B, Qayed M, McCracken C, et al. Phase II trial of costimulation blockade with abatacept for prevention of acute GVHD. J Clin Oncol. 2021;39(17):1865-1877. doi:10.1200/JCO.20.01086
Zeiser R, Polverelli N, Ram R, et al; REACH3 Investigators. Ruxolitinib for glucocorticoid-refractory chronic graft-versus-host disease. N Engl J Med. 2021;385(3):228-238. doi:10.1056/NEJMoa2033122