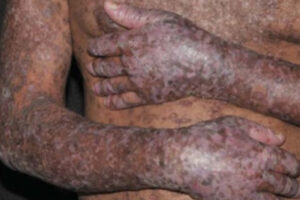
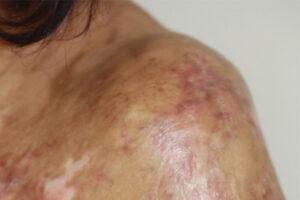
Oncology
Chronic Graft-versus-Host Disease
Optimizing the Chronic Graft-versus-Host Disease Treatment Paradigm
Overview
The prevention and treatment of chronic graft-versus-host disease (cGVHD) remain an unmet need, with steroid-refractory cGVHD representing a particular challenge. However, changes to the treatment paradigm are expected; numerous clinical trials are underway and a number of potential treatments are awaiting regulatory action.
What are some gaps in the current cGVHD treatment paradigm, and where do you see things going in the future?
Daniel R. Couriel, MD, MS
|
|
“As additional active agents become available for cGVHD, the goal of trying to spare patients from having to take corticosteroids will be on everyone’s mind.”
The next several years will be exciting because we will be learning how to use the new agents coming down the road. As additional active agents become available for cGVHD, the goal of trying to spare patients from having to take corticosteroids will be on everyone’s mind. My hope is that the changes that we will see over the next several years will have a very positive impact on patient morbidity and survival outcomes.
At the moment, we do not have established biomarkers that predict severity of cGVHD or response to therapy. In practice, we follow the patient clinically to assess the extent of ongoing cGVHD and the response to therapy. The assessment can be complex in that your response is not necessarily tied to any single organ or aspect of cGVHD, as reflected in the 2014 National Institutes of Health cGVHD consensus update. The burden of the whole syndrome of cGVHD also has components that have to do with treatment (eg, long-term corticosteroid use), and adverse effects such as diabetes and osteoporosis, among others, are essentially incorporated or blended into the total burden of cGVHD.
In addition to systemic therapy, we have learned that adjunctive therapy is important to the patient’s comfort. So, for example, if you have dry eye, your best treatment might be scleral lenses, which, of course, is not systemic. But how those scleral lenses may actually spare the use of corticosteroids or other therapies is a very interesting and ongoing question. Does using ancillary and supportive care spare the need for systemic therapy? That is a key question that has yet to be answered.
Corey Cutler,, MD, MPH, FRCPC
|
|
“With newer agents emerging, we will need to learn whether there are patient characteristics or phenotypes to suggest that a particular agent will be better than another in a given situation.”
The cGVHD treatment paradigm will likely change in the next few years. The prevention of cGVHD is not yet fully realized. We are using more regimens that incorporate post-transplant cyclophosphamide into the cGVHD prevention strategy, which may reduce the overall incidence of cGVHD, and we are testing additional strategies for prevention now.
We should learn the findings of the INTEGRATE study soon, which is evaluating the combination of corticosteroids and ibrutinib in newly diagnosed patients with cGVHD. We are also following other ongoing trials, such as the ROCKstar study (NCT03640481). We were excited to see the recent US Food and Drug Administration approval of belumosudil for cGVHD after failure of at least 2 prior lines of systemic therapy. I suspect that we may soon have additional agents available to choose from in the steroid-refractory setting as well. With newer agents emerging, we will need to learn whether there are patient characteristics or phenotypes to suggest that a particular agent will be better than another in a given situation. The challenge will be to determine which drug is the best choice for an individual patient. At some point, we will need to have head-to-head trials and trials of the therapeutic combinations of interest. With newer drugs coming, it is likely that the space is going to get even more crowded, which is only a good thing for our patients, and I suspect that the landscape will be markedly different in 3 to 5 years.
Sergio A. Giralt, MD
|
|
“The patient’s individual path to cGVHD will differ, and these differences will all need to be explored. Consider, for example, a patient who has had a haploidentical transplant with post-transplant cyclophosphamide, who we now see, perhaps post cellular therapies, with severe cGVHD.”
I think that cGVHD will be a topic of intense controversy and discussion over the next few years. The types of patients with cGVHD that we will be seeing will change because the GVHD prevention strategies that we are currently using are different from those used previously, and the stem cells, transplant protocols, and treatments that we are now using have also changed. The patient’s individual path to the cGVHD will differ, and these differences will all need to be explored. Consider, for example, a patient who has had a haploidentical transplant with post-transplant cyclophosphamide, who we now see, perhaps post cellular therapies, with severe cGVHD.
With the availability of perhaps several new drugs within the next year, we will need to learn which drug to use first and how to sequence them. Without clinical trials to inform therapeutic decision making, we will have to rely on common sense and experience based on patient comorbidities and known treatment side effects. Our approach to supportive care will also change in an effort to reduce patient morbidity.
References
Cain DW, Cidlowski JA. Immune regulation by glucocorticoids. Nat Rev Immunol. 2017;17(4):233-247. doi:10.1038/nri.2017.1
Crossland RE, Perutelli F, Bogunia-Kubik K, et al. Potential novel biomarkers in chronic graft-versus-host disease. Front Immunol. 2020;11:602547. doi:10.3389/fimmu.2020.602547
Inamoto Y, Martin PJ, Flowers MED, et al. Genetic risk factors for sclerotic graft-versus-host disease. Blood. 2016;128(11):1516-1524. doi:10.1182/blood-2016-05-715342
Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21(3):389-401.e1. doi:10.1016/j.bbmt.2014.12.001
Jagasia M, Lazaryan A, Bachier CR, et al. ROCK2 inhibition with belumosudil (KD025) for the treatment of chronic graft-versus-host disease. J Clin Oncol. 2021;39(17):1888-1898. doi:10.1200/JCO.20.02754
Partanen J, Hyvärinen K, Bickeböller H, et al. Review of genetic variation as a predictive biomarker for chronic graft-versus-host-disease after allogeneic stem cell transplantation. Front Immunol. 2020;11:575492. doi:10.3389/fimmu.2020.575492
Peled JU, Gomes ALC, Devlin SM, et al. Microbiota as predictor of mortality in allogeneic hematopoietic-cell transplantation. N Engl J Med. 2020;382(9):822-834. doi:10.1056/NEJMoa1900623
Penack O, Marchetti M, Ruutu T, et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2020;7(2):e157-e167. doi:10.1016/S2352-3026(19)30256-X
Solomon SR, Sizemore CA, Ridgeway M, et al. Corticosteroid-free primary treatment of chronic extensive graft-versus-host disease incorporating rituximab. Biol Blood Marrow Transplant. 2015;21(9):1576-1582. doi:10.1016/j.bbmt.2015.04.023
Waller EK, Miklos D, Cutler C, et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy: 1-year update of a phase 1b/2 study. Biol Blood Marrow Transplant. 2019;25(10):2002-2007. doi:10.1016/j.bbmt.2019.06.023
Williams KM, Inamoto Y, Im A, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2020 Etiology and Prevention Working Group report. Transplant Cell Ther. 2021;27(6):452-466. doi:10.1016/j.jtct.2021.02.035
Zeiser R, Polverelli N, Ram R, et al. Ruxolitinib (RUX) vs best available therapy (BAT) in patients with steroid-refractory/steroid-dependent chronic graft-vs-host disease (cGVHD): primary findings from the phase 3, randomized REACH3 study [abstract 77]. Abstract presented at: 62nd American Society of Hematology Annual Meeting and Exposition; December 5-8, 2020.